And Z particles are iron pieces. Class 7 Inside Our Earth.
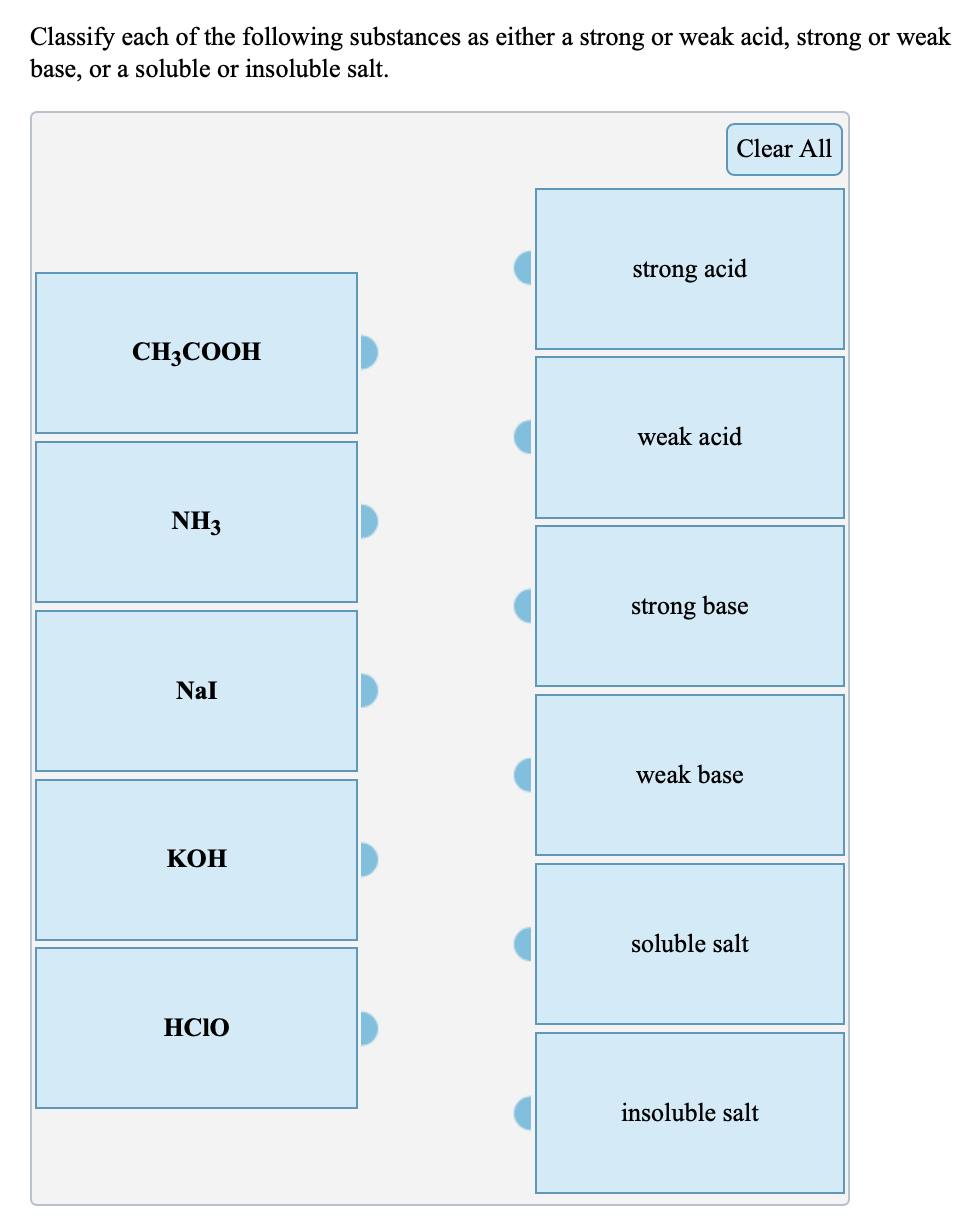
Solved Classify Each Of The Following Substances As Either A Chegg Com
A Na 2 Saq ZnCl 2.

. The salts of Group 1 metals are solubl e Li₂CO₃ and KOH. The Difference Between Soluble Substances and Insoluble Ones. Identify the following substances as pure substances elements compounds mixtures or solutions answers may include more than one classification.
Mud copper salt water carbon monoxide. Potassium sulfate or K_2SO_4 si soluble because all sulfates are soluble with the exception of those formed with silver calcium barium mercury lead and strontium ions FInally copper II hydroxyde or CuOH_2 is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium. Up to 24 cash back 21At standard pressure which substance becomes less soluble in water as temperature increases from 10C to 80C.
Hence the right answer is option A AlBr 3. It might look like its simply disappeared but in fact its still there - its just mixed in to form a liquid called a solution. Experts are tested by Chegg as specialists in their subject area.
Identify which of the following substances is an insoluble salt. Who are the experts. 2 Insoluble substances cannot form a 010-molar solution at 25 C.
This suggests presence of Aluminium ion in contrast to Sodium ion as Aluminium hydroxide formed by the two reactions give a white precipitate. 1 Soluble substances can form a 010-molar solution at 25 C. We review their content and use your feedback to keep the quality high.
A soluble substance is one that dissolves in a liquid usually water. Identify which of the following substances is an insoluble salt. Insoluble - insoluble less than 001g per 100g of water not exist - do not exist in the aqueous environment.
View solution View more. Insoluble most hydroxide compounds are insoluble d CaF 2-Insoluble most compounds containing F ion are insoluble e ZnCl 2 Soluble most chloride compounds are soluble f NiClO 3 2 Soluble most chlorate compounds are soluble 8 Burdge 922 Write total ionic and net ionic equations for the following reactions. So solubilitysoluble is the ability of a solute to dissolve in a solvent.
Thus A contains Aluminium ion. Solute is a substance that is soluble in a solvent in order to form a solution. She wants to know whether these substances are soluble in water or not.
However there are other definitions of solubility since a third term called slightly soluble is one that some in chemistry prefer to use. X particles are very heavy insoluble non-magnetic and contribute 50 of the mixture. Sugar salt mustard oil sand sawdust honey chalk powder petals of a flower soil copper sulphate crystals glucose wheat flour are some substances given to Paheli.
Solutes can be in liquid gaseous or solid phase. Which of the following gas is insoluble in water. They are of the same size cubical in shape and yellow in colour.
A mixture contains three different substances X Y and Z. Therefore we are only looking at BaSO4 MgSO4 Na2SO4 and NH42 SO4 as potential sulfate salts that are insoluble. Solvents can be in a liquid gaseous or solid state.
This definition means there are only two categories. Apotassium nitrate Bsodium chloride Cpotassium chlorate Dammonium chloride 22A student tested the solubility of a salt at different temperatures and then used Reference Table g to identify the salt. The sulfate salt is SO4 2.
When a salt such as sodium chloride table salt dissolves in water its ionic lattice is pulled apart so that the individual sodium and chloride ions go into solution. Sulfates are usually soluble except for BaSO ₄. Climate Vegetation and Wildlife.
Try a variety of the following. NaCl s Naaq Cl-aq In practice many salts that are described as insoluble. CLASSES AND TRENDING CHAPTER.
Oil butter grease enamel paint nail polish 5. And is insoluble in Barium sulphate is insoluble in dilute HCl and it is a white precipitate it is formed 10 Alien chloride reacts with sulphuric iron and sulphur it comes from salt solution are given salt as an iron and iron present in the given salt is added because sulphate ions are reacting with barium chloride solution to a white precipitate of Barium sulphate which is insoluble in. Thus BaSO₄ is insoluble.
Y particles are very light insoluble non-magnetic and contribute 40 of the mixture. The solubility of insoluble substances changes depending on the nature of. Solvent is a substance with dissolving capability thus can dissolve another substance.
Sorting materials into groups - Quiz. The green colour seen in firework displays is due to the chloride salt of. And a sulfate salt is a salt that has a cation with SO4 as the anion.
Salt is obtained from sea water by using which of the following process. K Na Li Ba 2 Ca 2 Mg 2 NH 4 Ag Mn 2 Fe 2 Co 2 Ni 2 Cu 2 Zn 2 Pb 2 Hg 2 Al 3 Cr 3 Fe 3 H I-KI. Identify the following substances if it is SOLUBLE or INSOLUBLE MISCIBLE or IMMISCIBLE to water.
Phosphates are insoluble Mg₃ PO₄₂ The two insoluble compounds are BaSO₄ and Mg₃ PO₄₂. Soy sauce marinade vinegar liquid food coloring 3. Sand and pebbles Stones and rocks 4.
Sodium chloride salt MSG vetsin Sucrose sugar and super white 2. If an insoluble salt forms by the reaction of soluble substances in water and falls out of solution we call it a precipitate see image. Also Aluminium hydroxide is soluble in strong base sodium hydroxide while it is insoluble in weak base ammonium hydroxide.
Class 6 Maps Practical Geometry Separation of Substances Playing With Numbers India. Help her in identifying soluble and insoluble substances in water. 1 Identify the non lustrous substance from the following option a Wood b Gold c Diamond d Platinum 2 Sugar and salt are the examples of a Soluble substances b Lustrous substances c Miscible liquids d In soluble substances 3 Arrangement of things in a proper order is called a Separation b Object c Sorting d Classification 4 sugar.
Ground black pepper.
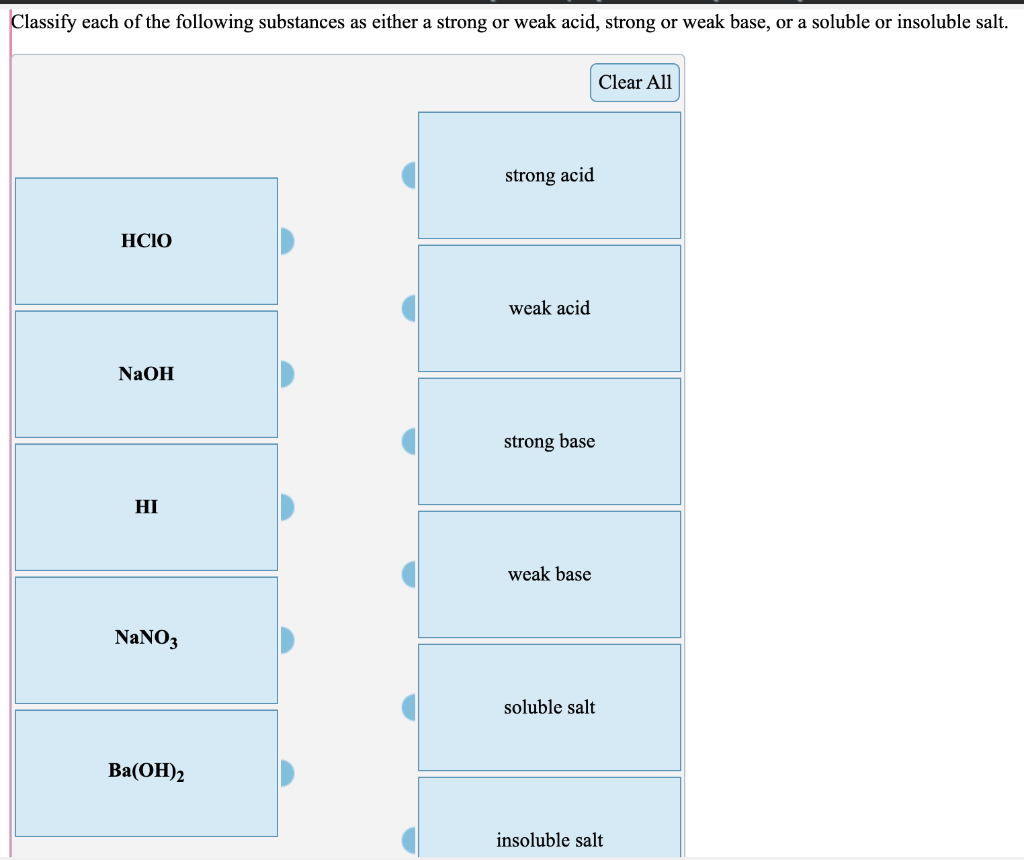
Solved Classify Each Of The Following Substances As Either A Chegg Com

Describe The Preparation Of Soluble And Insoluble Salts Preparation Salt Sodium Hydroxide

Soluble And Insoluble Substances Experiment Experiments Substances Fun
0 Comments